1
/
of
2
McKesson
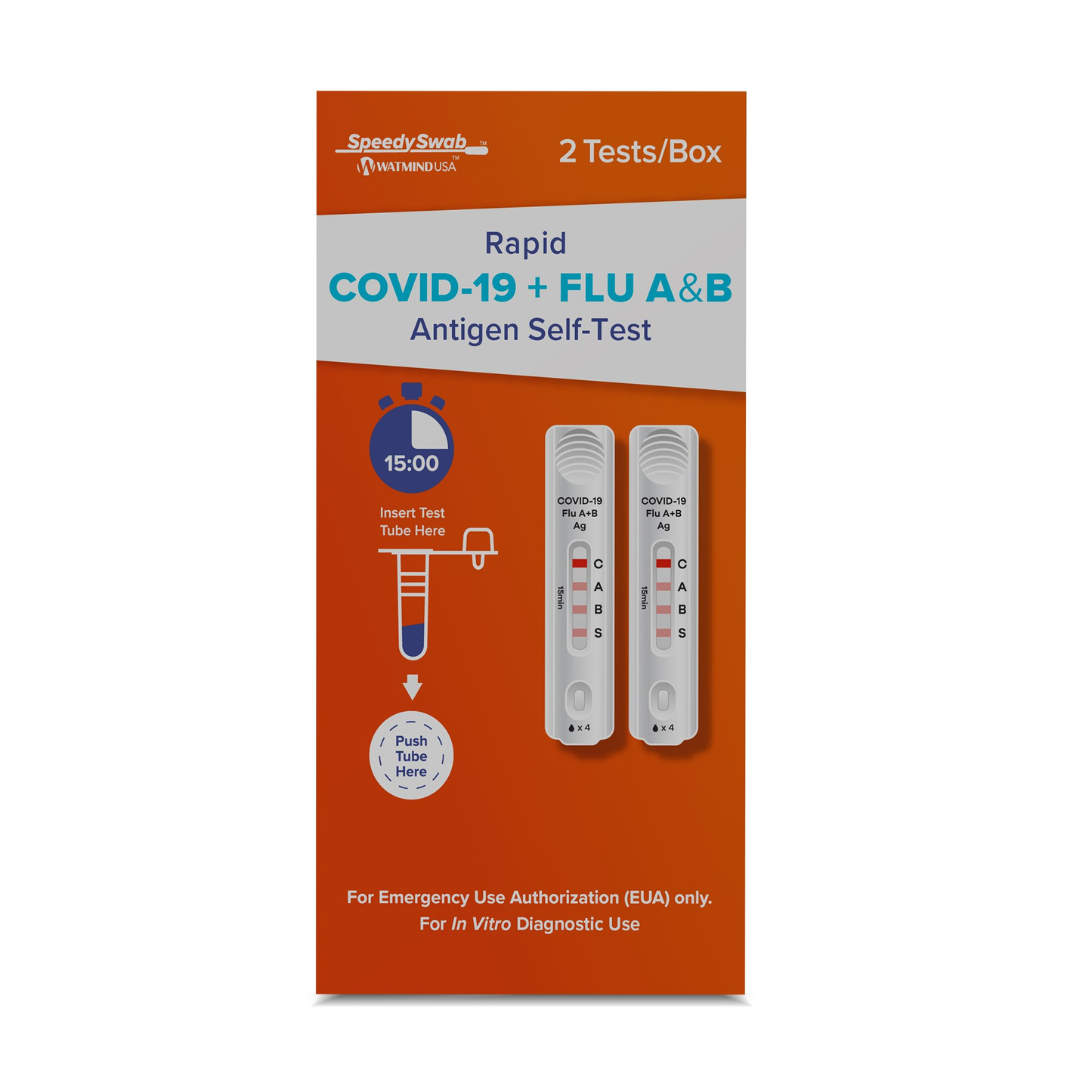
COVID-19 / Flu A+B Antigen Self-Test 2 Tests per Kit
COVID-19 / Flu A+B Antigen Self-Test 2 Tests per Kit
Regular price
$24.99 USD
Regular price
Sale price
$24.99 USD
Shipping calculated at checkout.
Quantity
Couldn't load pickup availability
Respiratory Test Kit SpeedySwab™
- The Speedy Swab Rapid COVID-19 + Flu A&B Antigen Self-Test is only for use under the Food and Drug Administration’s Emergency Use Authorization: https://www.fda.gov/medical-devices/covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2
- The Speedy Swab Rapid COVID-19 + FLU A&B Antigen Self-Test is a rapid, qualitative lateral flow immunoassay for the determination of the presence of SARS-CoV-2 and influenza A&B antigens in anterior nasal swab specimens
- The test strip in each device contains mouse monoclonal capture antibodies to the nucleocapsid protein (NP) of SARS-CoV-2, Influenza A and Influenza B and goat anti-Mouse IgG control antibody immobilized in the test and control regions on the nitrocellulose membrane, respectively
- This test is authorized for non-prescription home use with self-collected anterior nasal swab specimens from individuals aged 14 years or older, or with adult-collected anterior nasal swab specimens from individuals two (2) years or older
- This test is only authorized for individuals with signs and symptoms of respiratory infection consistent with COVID-19 within the first five (5) days of symptom onset when tested at least twice over three days with at least 48 hours between tests
- Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status
- Positive results do not rule out bacterial infection or co-infection with other viruses
- Individuals who test positive with the Speedy Swab Rapid COVID-19 + Flu A&B Antigen Self-Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary All negative results are pres
- All negative results are presumptive and confirmation with a molecular assay, if necessary for patient management, may be performed
- Negative results do not rule out SARS-CoV-2, influenza A, and influenza B infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions such as isolating from others and wearing masks
- Negative results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with SARS-CoV-2, influenza A, and influenza B infection
Share